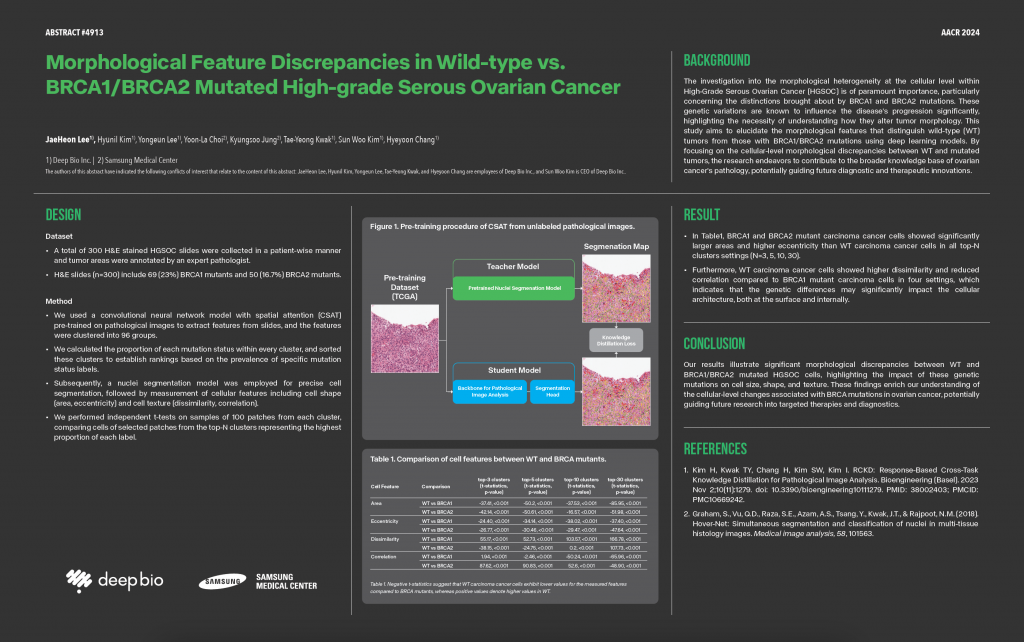
Deep Bio Inc., a leader in AI-driven digital pathology solutions, announced the publication of an external validation study for its DeepDx Prostate AI algorithm, conducted by Stanford Medicine.
The study, titled “External Validation of an Artificial Intelligence Model for Gleason Grading of Prostate Cancer on Prostatectomy Specimens,” was published in the British Journal of Urology International.
SEOUL, SOUTH KOREA, August 6, 2024
The study evaluated the performance of DeepDx Prostate in detecting and grading prostate cancer on whole-mount radical prostatectomy (RP) specimens. The DeepDx Prostate algorithm, which had been originally trained and validated on prostate core needle biopsy (CNB) images from two hospitals in South Korea, was tested to determine its generalizability to different patient populations and much larger prostatectomy specimens. Researchers aimed to assess the model’s performance on RP specimens from an institution it had never encountered before, without any fine-tuning. The validation study demonstrated the following key findings:
1. High Accuracy: The algorithm achieved an impressive sensitivity of 0.997 and specificity of 0.88 in detecting cancer presence in radical prostatectomy specimens.
2. Agreement with Pathologists: The AI showed strong agreement with expert uropathologists, with Cohen’s Kappa values of UWK 0.91 for cancer presence, QWK 0.89 for Gleason grade classification, and QWK 0.89 for risk group identification (benign, low [GG1], intermediate [GG 2-3], and high-risk [GG 4-5]).
3. Generalizability: The algorithm demonstrated robust performance across different datasets and tissue types, highlighting its potential for widespread implementation across various healthcare settings.
Sun Woo Kim, CEO of Deep Bio, commented, “Accurate grading of Gleason scores is key to optimizing treatment plans and predicting outcomes. The swift and precise results provided by DeepDx Prostate will be invaluable in clinical practice.”
Bogdana Schmidt MD, MPH, the lead author of this validation study and current assistant professor at the University of Utah, said, “The validation of DeepDx Prostate through this study underscores its generalizability and potential for widespread clinical implementation. This tool will aid in the timely and accurate grading of prostate cancer, which is crucial for developing effective treatment plans.”
The study concludes that DeepDx Prostate algorithm is an accurate tool for identifying and grading prostate cancer on digital histopathology images of whole-mount RP specimens, demonstrating almost perfect concordance with expert GU pathologists and impressive performance in various clinically relevant tasks.
Deep Bio Inc. is committed to advancing AI technology to enhance diagnostic accuracy and efficiency in pathology. When used for its intended tissue type—CNB samples—DeepDx Prostate demonstrates 99% sensitivity and 97% specificity.
About Deep Bio
Established in 2015, Deep Bio Inc. is a medical AI development company specializing in deep learning and cancer pathology diagnostics. The company develops in-vitro diagnostic medical device software (IVD SaMD) that enhances the accuracy of cancer diagnosis and prognosis, aiding in better treatment decision-making.
Deep Bio’s prostate cancer diagnostic AI solution, which has obtained the European CE-IVD certification, analyzes high-resolution Whole Slide Images (WSI) to identify and segment cancerous lesions. The software classifies each lesion by histological type or risk grade, measures lesion size, and provides various metrics, such as the proportion of each lesion type and the overall tissue lesion ratio, essential for cancer diagnosis, prognosis, and treatment planning.
The AI solution provides detailed analysis results and reports, improving patients’ and professionals’ accessibility to medical information. This innovation earned Deep Bio the CES Innovation Award in 2024.
![[Peer-Reviewed Publications] Prostate International – Artificial intelligence–driven digital pathology in urological cancers: current trends and future directions](https://deepbio.co.kr/wp-content/uploads/2024/02/2949012065_mn7tqI3w_249209586711fde517f0bc3e58cb687efa1afe74.png)
![[DP&AI Congress 2025] Deep Bio to Participate in the 11th Digital Pathology & AI Congress: Europe](https://deepbio.co.kr/wp-content/uploads/2023/12/thumb-2949012065_f4FSHsq0_ca04d97dcf2579261bf285cb90e0a5a0af54e263_1400x787.png)