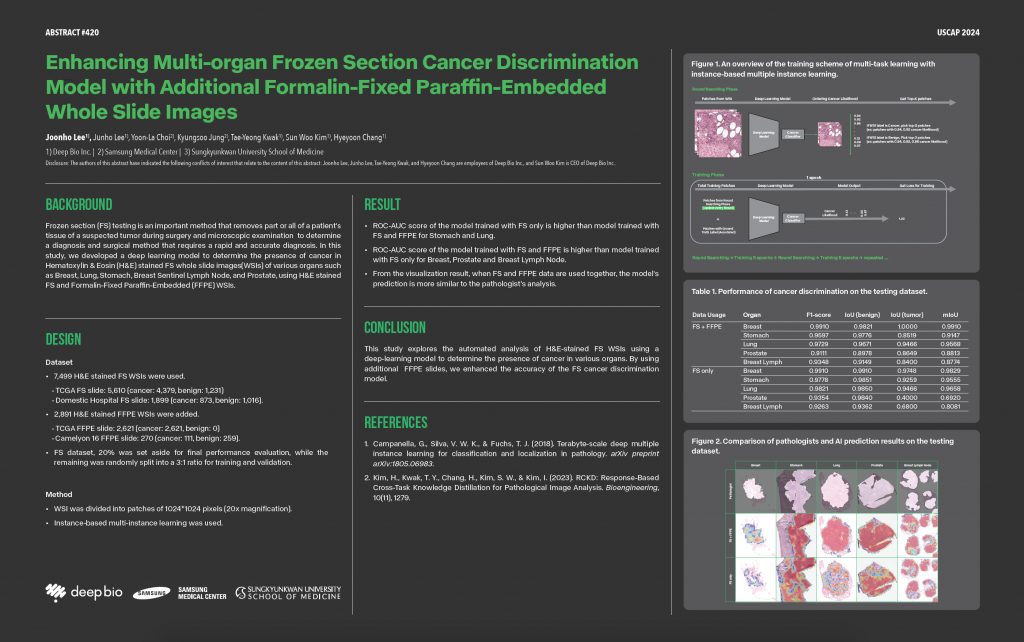
South Korea based Deep Bio, a pioneering artificial intelligence (AI) healthcare firm focused on cancer pathology, is making waves in the industry. Their recent involvement in the innovative CancerX initiative, part of the White House Cancer Moonshot programme, marks a pivotal moment. Spearheading advancements in deep learning and cancer pathology, Deep Bio aims to revolutionise cancer diagnosis and prognosis. Sun Woo Kim, CEO, Deep Bio sheds light on their transformative mission, AI-driven healthcare, data privacy, among others.
What inspired Deep Bio to focus on developing in vitro diagnostic software for cancer pathology?
With the advancement of AI like deep learning, AI can distinguish images and classify them for the first time, just as the human eyes can. I found that cancer detection and diagnosis were very subjective at that time. Pathologists have difficulty providing the exact tumour measurements using a microscope because human pathologists estimate the tumour area and provide the proportion of cancer information. Moreover, interobserver and intraobserver variability in the grading of tumours can impact therapy selection and patient outcomes. It would be good to apply AI technology for cancer pathology for accuracy and consistency.
How does Deep Bio leverage deep learning in its in vitro diagnostic software for cancer pathology?
Identifying diverse tissue morphological patterns related to tumour malignancy, differentiation levels, and prognosis in cancer pathology is crucial.
Deep learning proves highly effective in recognising specific patterns within large datasets, as demonstrated by its surpassing human recognition in the ImageNet challenge.
Leveraging this capability, Deep Bio is developing in vitro diagnostic software for cancer pathology. This software employs deep learning-based image analysis for various tasks, such as identifying tissues and cancerous lesions, distinguishing between cell nuclei and cell membranes, classifying and grading histologic tumour types, estimating gene mutations, and predicting patients’ prognoses.
The goal is to enhance the precision and efficiency of cancer diagnostics by harnessing the power of deep learning to analyse intricate tissue patterns and provide valuable insights into various aspects of cancer pathology.
What are some of the challenges that Deep Bio has faced in developing and implementing its AI healthcare solutions?
The efficacy of deep learning hinges on access to substantial datasets to achieve high accuracy. However, acquiring such extensive datasets challenges medical data and sensitive personal information.
This difficulty is compounded by the intricate nature of obtaining significant annotation data from pathology experts for various organs and cancer types. The demand for pathological diagnosis is rising while the number of pathologists is declining, exacerbating the challenge of building comprehensive datasets.
Complicating matters further is the issue of inter-observer variability, making both data collection and performance evaluation exceptionally challenging. To address these complexities, our diagnostic AI applications undergo rigorous comparisons with pathologists, measuring accuracy and speed.
The expectation is that these AI applications will match and surpass pathologists in terms of accuracy and speed, meeting user expectations. Achieving this while ensuring cost-effectiveness poses a formidable engineering task, requiring innovative solutions to overcome the hurdles associated with limited data access, inter-observer variability, and the evolving landscape of pathology demands.
What collaborative efforts does Deep Bio engage in with healthcare providers or institutions to implement and refine its technology solutions?
Deep Bio’s medical AI solutions are developed based on extensive medical data and expert knowledge. Collaborating with healthcare professionals, particularly pathologists, is indispensable for developing and delivering optimal solutions. We engage closely with domestic and international healthcare institutions and pathologists to ensure compliance with data regulations, construct essential datasets, assess performance, and pinpoint areas for refinement. Our principal collaboration entails partnering with providers of digital pathology platforms to ensure the stable delivery of our solution and perpetually enhance its efficacy through resolving engineering challenges.
How does Deep Bio address concerns regarding data privacy and security when dealing with sensitive patient information?
Deep Bio implements strict data security measures to prevent unauthorised access to medical data. Our research systems operate on segregated networks, and access to research data is restricted to designated researchers; We hold ISO 27001 certification for our information security management system.
Deep Bio’s solutions feature robust security measures in line with the South Korean Ministry of Food and Drug Safety (MFDS) cybersecurity checklist. For example, all data communication is encrypted, and unauthorised access attempts are promptly blocked. Our cloud services comply with HIPAA regulations, ensuring secure storage, transmission, and processing of protected health information (PHI).
How does Deep Bio perceive the current and future trends in AI-driven healthcare, particularly in the context of cancer pathology?
Deep Bio perceives the current and future trends in AI-driven healthcare, particularly in cancer pathology, amid a notable shift from analog to digital pathology. This transformation involves moving from traditional glass slide reviews to evaluating digitised images captured by high-definition scanners viewed on computer monitors.
This change allows pathologists to conduct remote reviews, fostering accessibility for patients in underserved areas. Concurrently, it fuels the development of AI-assisted pathology, utilising digitised images to train algorithms that assist pathologists in making more accurate diagnoses, prognoses, and predictions of therapeutic responses.
Deep Bio anticipates digitising nearly all pathology cases in the envisioned future. These digitised images would play multifaceted roles, serving as pre-screen analyses before pathologist reviews, real-time support during consulting reviews, or post-sign-out quality control (QC) reviews to detect misdiagnoses or discrepancies. This trajectory reflects a broader trend of seamlessly integrating AI into healthcare workflows, enhancing diagnostic precision, efficiency, and accessibility in cancer pathology.
Are there any upcoming projects or products that you are particularly excited about?
We believe that AI will be integrally involved in not only the diagnosis of cancer but also in determining the prognosis for the patient and predicting the best therapeutic option – essentially, the realm of precision medicine. In the case of prostate cancer, many men do not require definitive treatment and are candidates for surveillance. AI can be used to identify those cases that may require more definitive therapy versus those that can be safely monitored. Much of this will be done based on the analysis of morphologic images captured from the hematoxylin and eosin (H&E) stained slides. In addition, the future holds a more comprehensive integration of AI, incorporating additional layers of information such as genetic sequencing data and population or metadata. This holistic approach aims to significantly improve the precision and effectiveness of diagnosis, prognosis, and predictive medicine.
As for our expansion plans, our DeepDx Prostate product is CE-marked for distribution throughout the European Union. In addition, our DeepDx Prostate product is available through our channel partner’s image management systems (IMS). Many image management vendors are already embedded within hospitals and pathology labs worldwide. So, one mechanism for widespread adoption is to make our algorithms available across multiple platforms. We currently have various customers in the United States that utilise our algorithm as a Laboratory Developed Test (LDT). These laboratories have rigorously validated our algorithm in their laboratories under Clinical Laboratory Improvement Amendments (CLIA). Over the next several months, we will add channel partners and expand our distributor network worldwide.
Ayesha Siddiqui
![[BioSpectrum Asia] AI will be integrally involved in not only the diagnosis of cancer but also in determining the prognosis and best therapeutic option](https://deepbio.co.kr/wp-content/uploads/2023/12/thumb-2949012065_f4FSHsq0_ca04d97dcf2579261bf285cb90e0a5a0af54e263_1400x787.png)